
RAMP COVID-19 Antigen Test Gets CE Mark
Response Biomedical launches COVID-19 (SARS-CoV-2) antigen test for countries accepting CE mark, to support the growing demand for testing. Response Biomedical Corp. (Response), a global

Home / News & Events

Response Biomedical launches COVID-19 (SARS-CoV-2) antigen test for countries accepting CE mark, to support the growing demand for testing. Response Biomedical Corp. (Response), a global
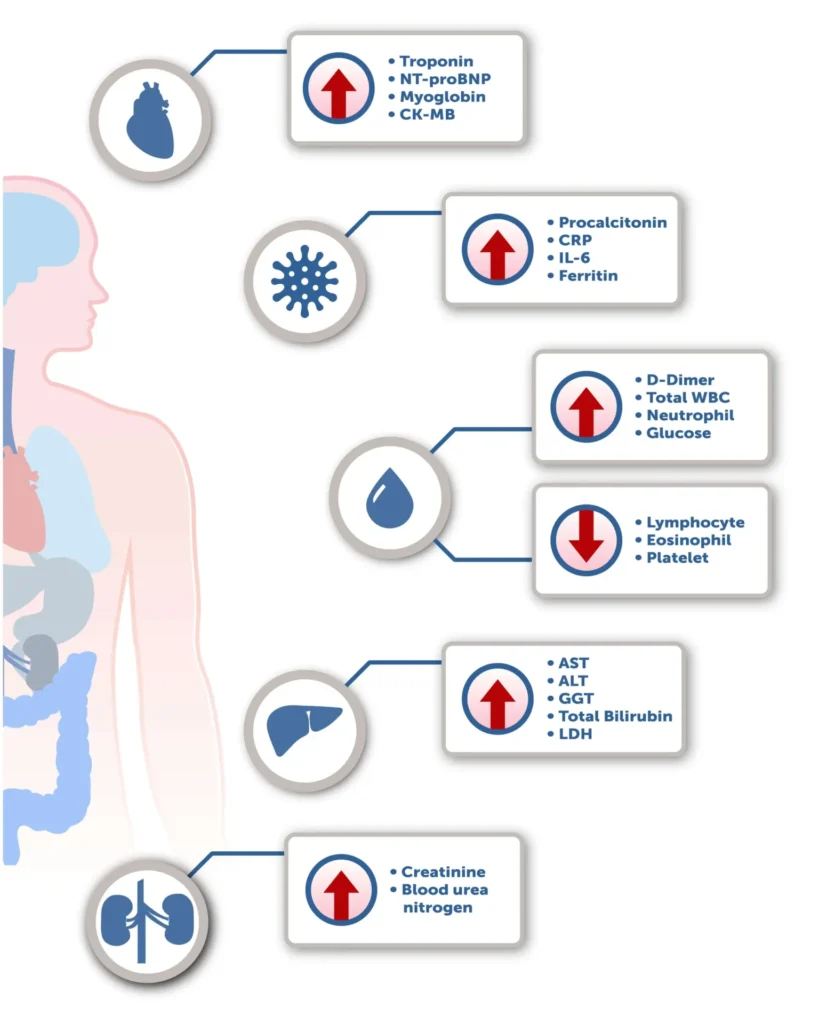
As a medical device manufacturer, we have been closely monitoring the research that is currently being conducted into the detection of SARS-CoV-2. Response Biomedical’s purpose

In order to provide our channel partners with the highest level of support and education, Response Biomedical Corp. recently implemented the RAMP Education And Development
Response Biomedical Corp. is proud to be part of Canada’s fight against COVID-19 by developing a COVID-19 Antibody (Serology) test on the RAMP® platform. The
24 Hour Technical Support Available for all RAMP Customers
Start Testing Today
Not all products are available in all regions. Contact us for availability in specific markets.
"*" indicates required fields
"*" indicates required fields